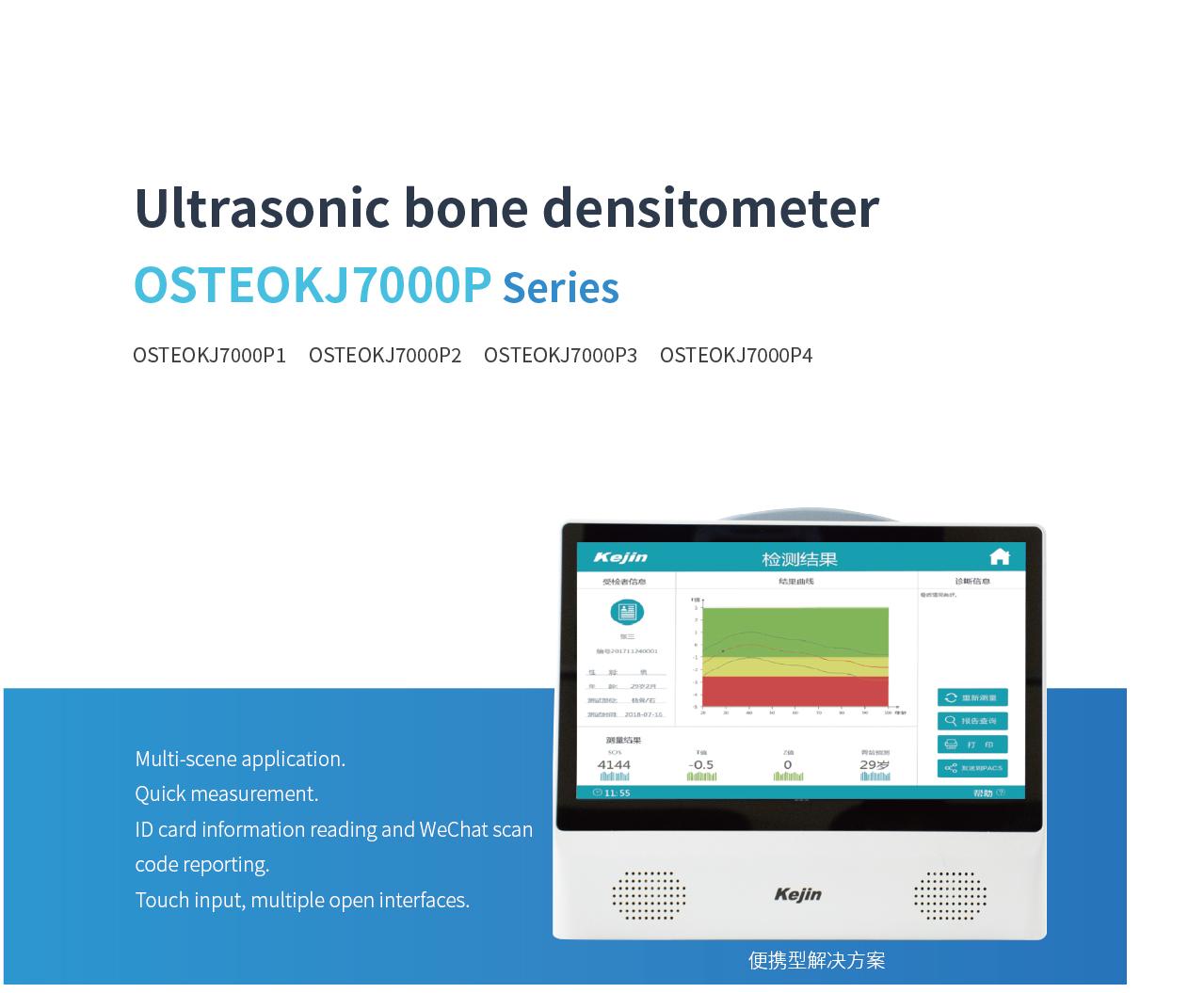
OSTEOKJ7000P Portable BMD

Product Parameters:
Product Name: BMD
Model: OSTEOKJ7000P1, OSTEOKJ7000P2, OSTEOKJ7000P3, OSTEOKJ7000P4
Brand: Kejin
Medical/Home Use: Medical
Domestic/Imported: Domestic
Manufacturer: Nanjing Kejin
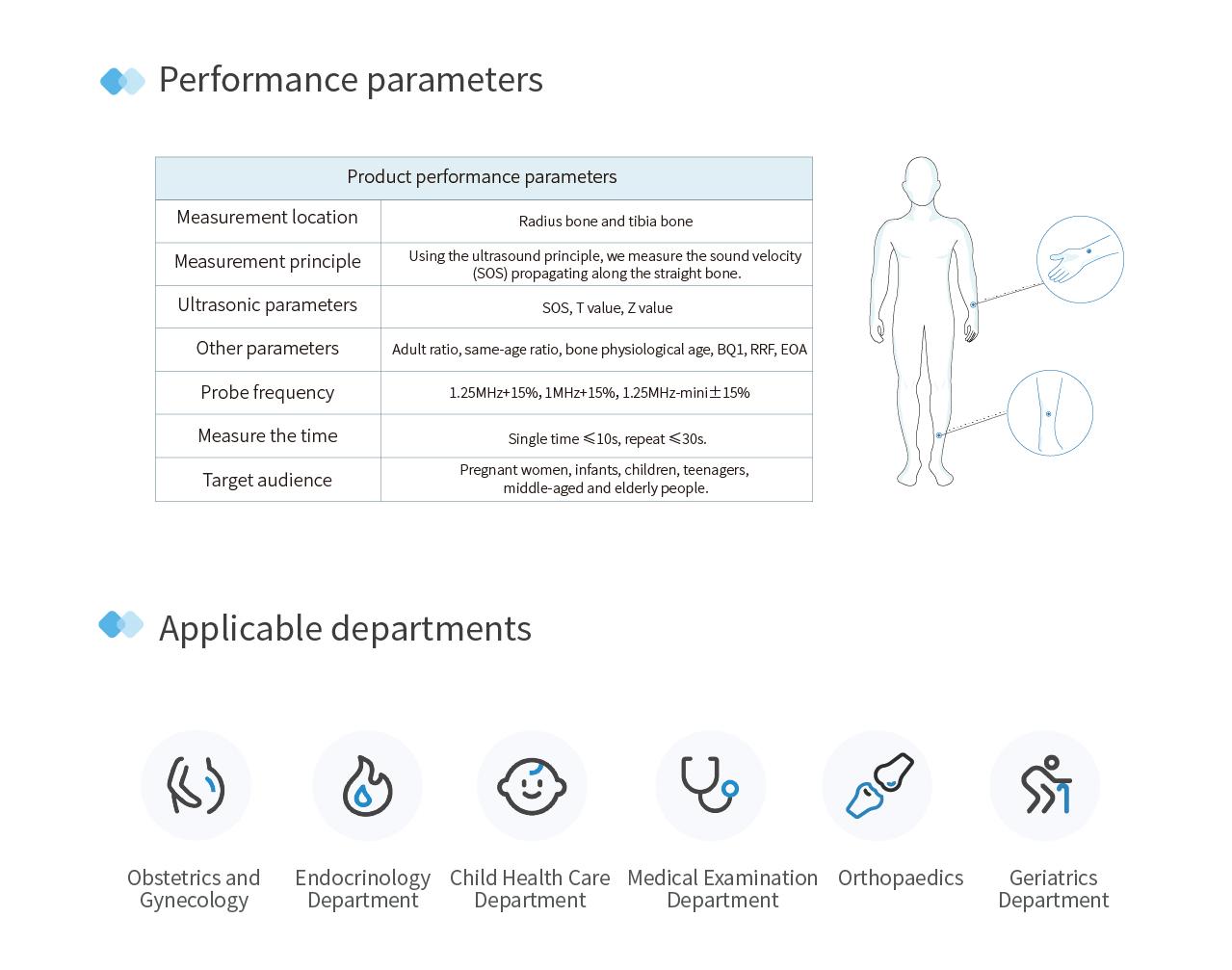
Scope of Application: Uses ultrasound to detect the bone density value of patella or phalanx.
OSTEOKJ7000P Series Portable BMD is a portable solution launched by Kejin. It uses axial reflection technology to measure the speed of sound (SOS) along the direction parallel to the patella or phalanx, and calculates a set of parameters to reflect bone quality.
Product Features
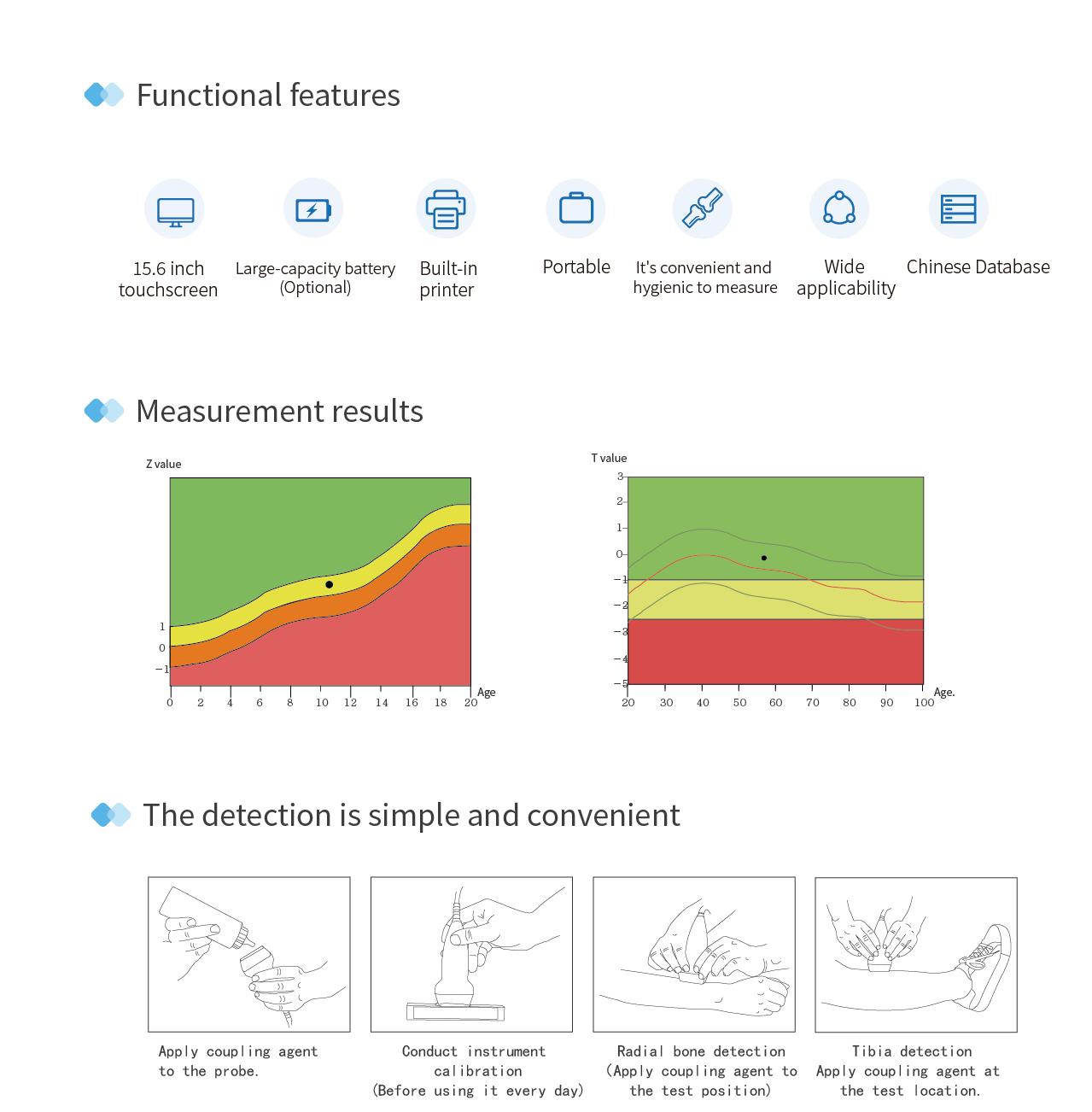
Touch Screen Operation
Equipped with a 15.6-inch touch screen for convenient operation.
Large Capacity Battery
Optional 19200mAh battery pack for longer endurance.
Built-in Printer
The BMD can output reports via built-in thermal printer or external printer.
Portable Design
Adopts an integrated portable design, suitable for multiple scenarios.
Multiple Open Interfaces
Supports ID card reading, WeChat QR code report, and can connect to hospital systems.
Chinese Database
Data source is based on statistical reference database of bone density values for Chinese population.
Product Functions
Bone Physiological Age
Comparison of measured bone mass with average bone mass of each age group
T-score, Z-score
Standard deviation of bone mass compared to average value of young adults of the same gender (T-score) and same age group (Z-score)
Child and Adult Detection
KJ-PW-1MHz probe is suitable for normal and slightly obese subjects; KJ-PW-1.25MHz probe is more suitable for normal subjects; KJ-PW-1.25MHz-mini probe is more suitable for infants and children.
Report Output
Subjects aged ≥20 years: Results display measurement date, T-score chart, comparison chart of T-score and Z-score with average values (orange bars represent the subject's T-score and Z-score, green bars represent the average), SOS, T-score, Z-score, adult ratio, peer ratio, bone physiological age, BQI, RRF, and EOA; result analysis shows the software's judgment on the subject's bone quality (normal, osteopenia, osteoporosis).
Subjects aged <20 years: Results display measurement date, SOS, Z-score, bone physiological age, peer ratio, Z-score chart, obesity chart, etc.; in the growth analysis list, values such as father's height, mother's height, current height, weight, reference height for each age, predicted height, and BMI are shown.
Applicable Departments
Obstetrics, Endocrinology, Pediatrics, Physical Examination, Orthopedics, Geriatrics
Note: The above description is excerpted from the product technical requirements and manual.
Medical Device Name: BMD
Medical Device Manufacturer: Nanjing Kejin
Medical Device Registration Certificate Number/Product Technical Requirements Number: SZC-20252070349
Medical Device Advertisement Approval Number: SGA-2025-007671
Warning: Please read the product manual carefully or purchase and use under the guidance of medical personnel. Forbidden content or precautions are detailed in the manual.