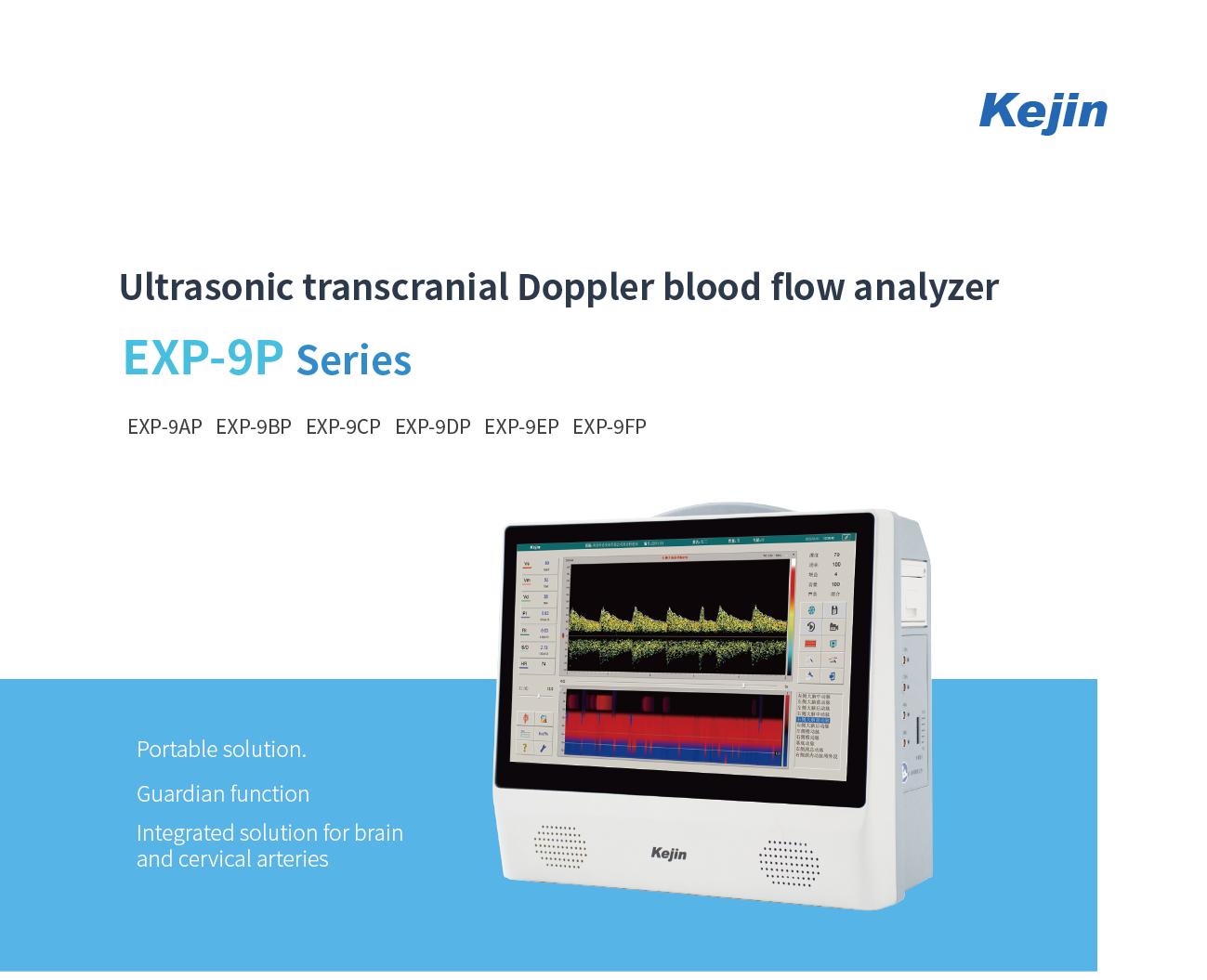
EXP-9P TCD

Product Parameters
Product Name: Transcranial Doppler Blood Flow Analyzer
Model: EXP-9P Series
Brand: Kejin
Medical/Home Use: Medical
Domestic/Imported: Domestic
Manufacturer: Nanjing Kejin
EXP-9P Series TCD is a dual-channel, nine-depth portable TCD solution launched by Kejin. It uses pulsed ultrasound to penetrate the thin part of the temporal bone to detect blood flow in the intracranial arteries. It can also use continuous ultrasound to detect blood flow in superficial vessels, quantitatively evaluating the blood supply status of cerebral and superficial vessels. It can perform embolus detection and bubble test, providing basis for diagnosis of various cerebral vascular diseases in terms of nature, degree, and scope.
The TCD is equipped with a monitoring probe and probe cover, which can realize monitoring function, mainly used for blood flow measurement of cerebral, cervical, and peripheral vessels.
Features
Touch Screen Operation
Equipped with a 15.6-inch touch screen, high-definition display, and more convenient touch operation.
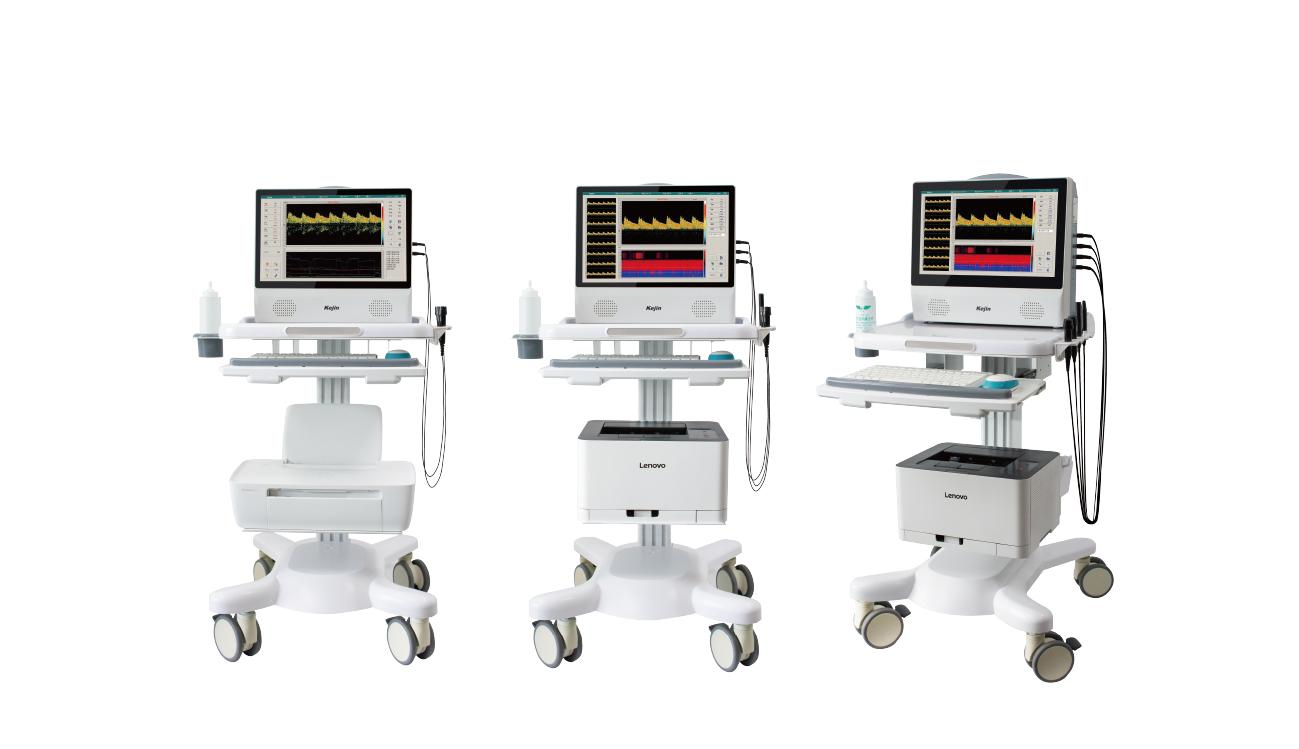
Built-in Printer
The product is equipped with a built-in thermal printer, which can print reports on site after testing. Users can also connect an external printer.
Large Capacity Battery
Optional 19200mAh large capacity battery pack for longer endurance.
Portable
Portable appearance design, suitable for multiple scenarios.
High Sensitivity Probe
Equipped with multiple frequency high sensitivity ultrasound probes and monitoring probes for quick vessel location.
QR Code Report
Supports obtaining test reports via WeChat QR code for more convenient online viewing.
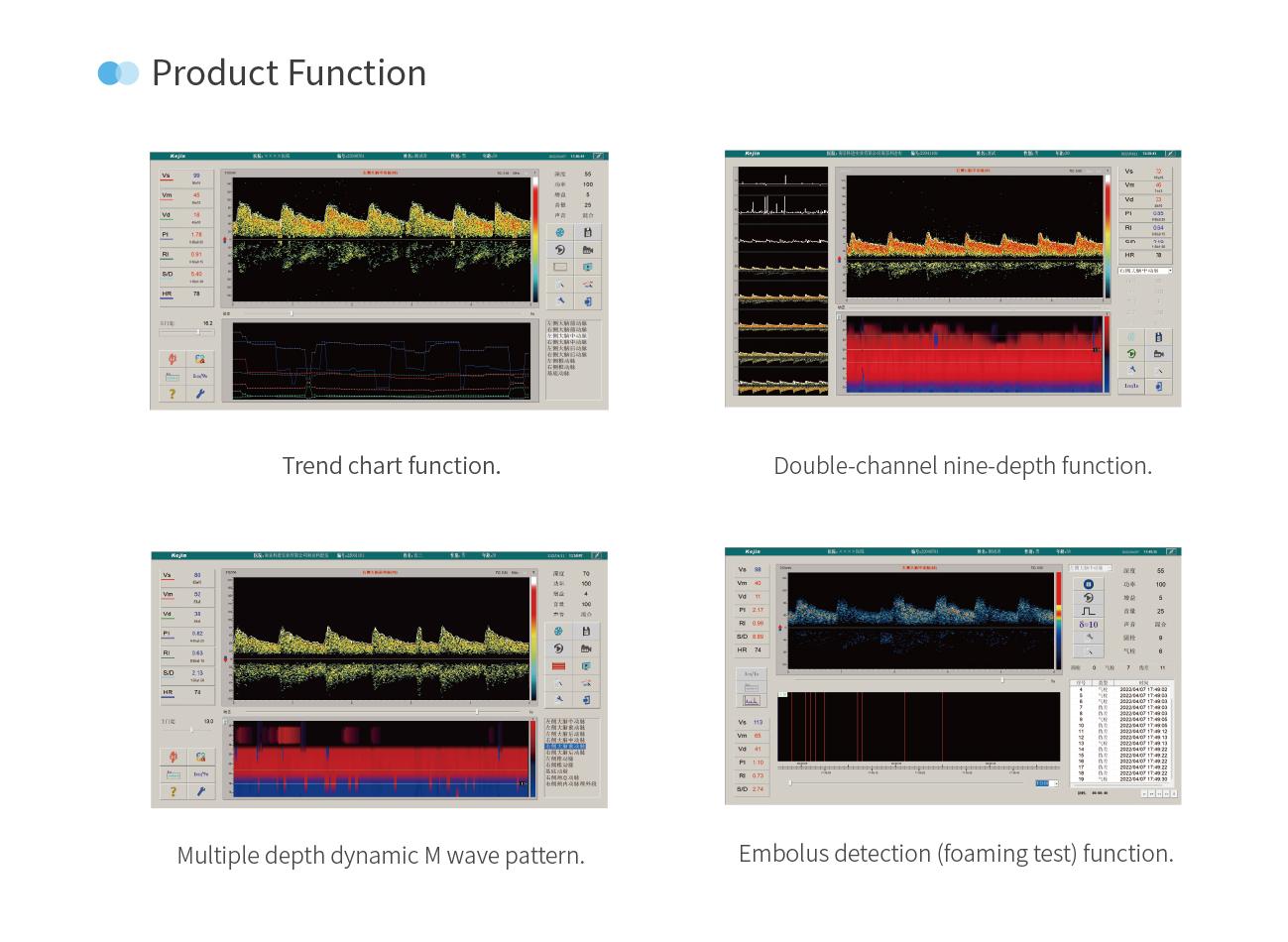
Product Functions
Trend Chart Display
Clicking the "M Wave Display Area" will change to a real-time trend chart of parameters, up to 7 different parameters can be displayed simultaneously.
Automatic Vessel Search
Vessel search technology can capture weak signals, locate the position of the heart vessel, shorten the time to find the vessel, and reduce dependence on operator skill.
Multi-depth Dynamic M-mode
After starting the measurement, the flow velocity of the vessel detected within the depth range will be automatically displayed.
Embolus Detection (Bubble Test) Function
Supports single-channel and dual-channel embolus detection modes, and can also perform apnea test and brain death test in the vessel selection area.
Scope of Application
Blood flow measurement of cerebral, cervical, and peripheral vessels
Detection of blood flow in intracranial arteries
Detection of blood flow in superficial vessels
Quantitative evaluation of cerebral vessel blood supply
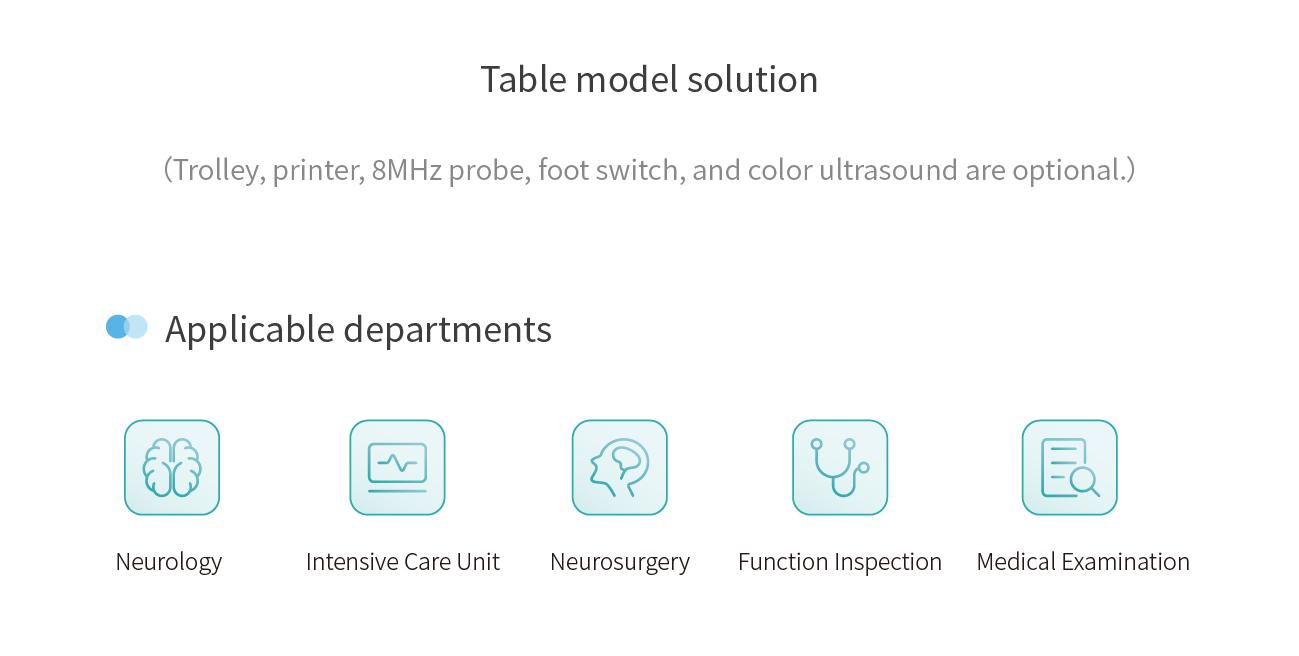
Applicable Departments
Neurology, ICU, Neurosurgery, Functional Examination, Physical Examination, etc.
Note: The above description is excerpted from the product technical requirements and manual.
Medical Device Name: Transcranial Doppler Blood Flow Analyzer
Medical Device Manufacturer: Nanjing Kejin
Medical Device Registration Certificate Number/Product Technical Requirements Number: SZC20252070292
Medical Device Advertisement Approval Number: SZG20251221-06299
Warning: Please read the product manual carefully or purchase and use under the guidance of medical personnel. Forbidden content or precautions are detailed in the manual.