OSTEOKJ3700S BMD


Product Name: OSTEOKJ3700S BMD
Product Parameters
Model: OSTEOKJ3700, OSTEOKJ3700S
Brand: Kejin
Medical/Home Use: Medical
Domestic/Imported: Domestic
Manufacturer: Nanjing Kejin
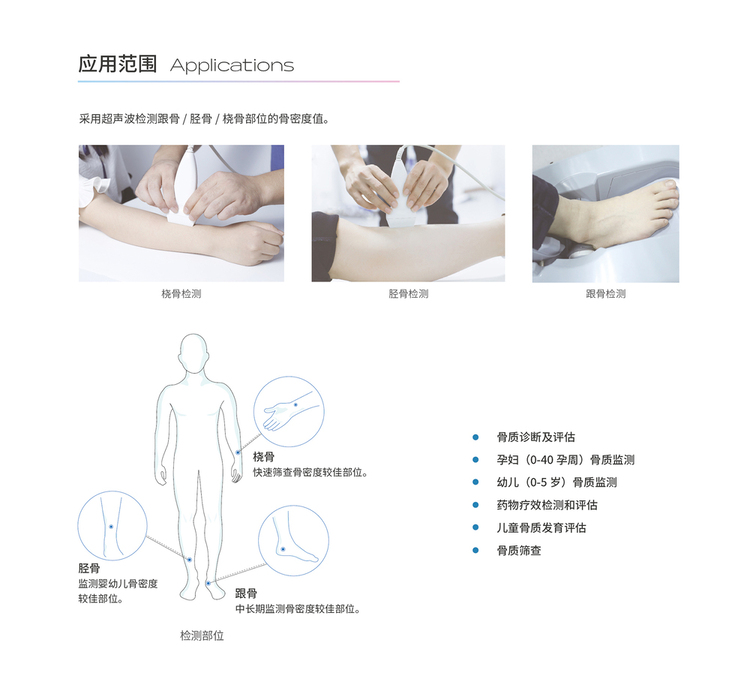
Measurement Site: Phalanx, Calcaneus, Patella
Product Introduction
Scope of Application
This product is suitable for bone density detection, using ultrasound to detect the bone density value of calcaneus/patella/phalanx.
Parameter Measurement, Calculation and Display
Calcaneus Mode: SOS, BUA, Osteo Index (OI), T-Score, Z-Score, T-Ratio, Z-Ratio, Bone Physiological Age, BMI, Predicted Height
Patella/Phalanx Mode: SOS, T-value, Z-value, T-Ratio, Z-Ratio, Bone Physiological Age, BQI, RRF, EOA, BMI, Predicted Height
Main unit continuous working time >8 hours, Bluetooth transmission distance not less than 5 meters.
Waterproof
Patella/Phalanx ultrasound probe waterproof level meets IPX7 requirements.
Recommended External Configuration
Normal use of this product requires external computer, printer and other equipment. Users can choose optional accessories for this product.
If users purchase and use by themselves, the purchased equipment should meet the requirements of GB9706.1-2020 and YY9706.102-2021 standards.
Data and Device Interface Description
Multi-site ultrasound BMD Windows software supports obtaining subject information from ID card reader;
Multi-site ultrasound BMD Android software supports obtaining reports via WeChat QR code, printing reports via network printer, wireless Bluetooth connection to main unit, and obtaining data from main unit.
Measurement Mode
In the pre-measurement preparation interface, you can choose "Single Site Mode" or "Multi-site Mode".
Measurable Sites: Left/Right Phalanx, Left/Right Patella, Left/Right Calcaneus. After selecting the measurement site, click "Start Measurement".
Patella/Phalanx Mode
During measurement, the interface will display subject information, real-time test curve, probe parallelism, parallelism setting, and measurement results.
Calcaneus Mode
During measurement, the interface will display subject information, real-time test curve, measurement time, measurement progress bar, and prompt "Note: Do not move the foot during measurement" for more accurate data.
When the subject is under 8 years old, the interface will play animation.
Note: The above description is excerpted from the product manual.
Medical Device Name: Ultrasound BMD
Medical Device Manufacturer: Nanjing Kejin
Medical Device Registration Certificate No./Product Technical Requirement No.: SuXieZhuan 20222071964
Medical Device Advertisement Approval No.: SuXieGuangShen (Wen) No. 251221-00208
Advice: Please read the product manual carefully or purchase and use under the guidance of medical staff. For contraindications or precautions, see the manual.