OSTEOKJ7600+ Wireless BMD
OSTEOKJ7600+ Wireless BMD
Product Parameters
Product Name: Wireless BMD
Model: OSTEOKJ7600 Series
Measurement Site: Radius/Tibia
Brand: Kejin
Medical/Home Use: Medical
Domestic/Imported: Domestic
Manufacturer: Nanjing Kejin
Scope of Application
Scope: This product uses ultrasound to detect the bone density value of the tibia or radius.
Product Introduction

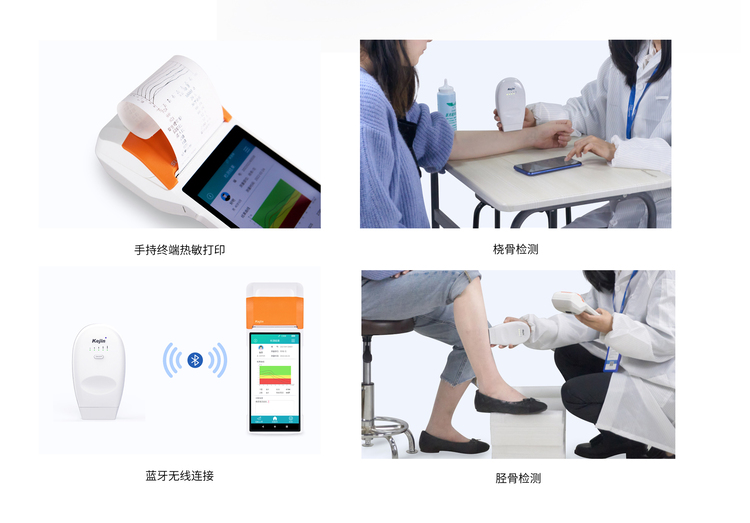
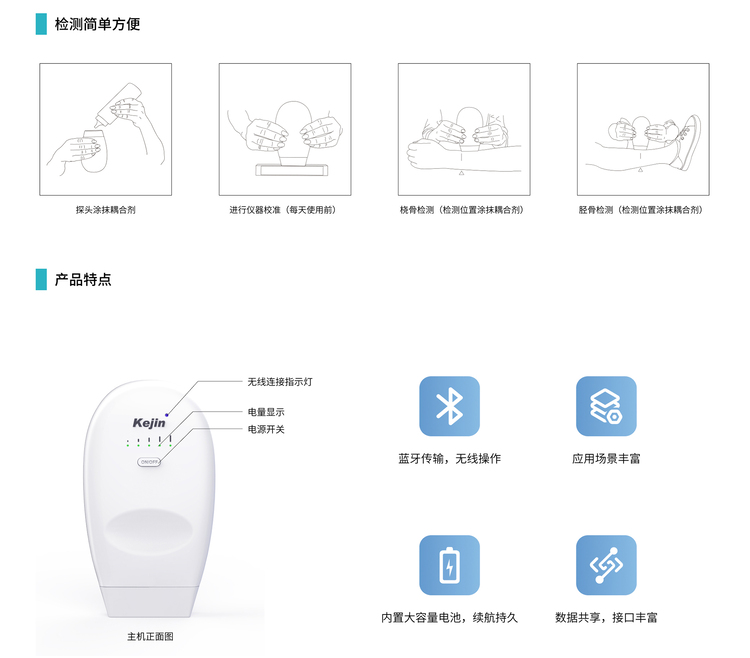

Model: OSTEOKJ7600, OSTEOKJ7600+, OSTEOKJ7600V, OSTEOKJ7600V+
External Configuration: The wireless BMD can connect to external terminals (such as computers, tablets, mobile phones, etc.) via Bluetooth.
Measurement Results:
After the measurement is completed, click "View Results" to enter the results interface.
The interface will display subject information, measurement values, and data charts. Users can assess osteoporosis risk by the position of the measurement point in the chart. Diagnostic analysis and additional diagnostic information can be added to the test results.
If the subject is under 20 years old, the interface will display an additional height prediction data compared to adults. In the test interface, you can also perform "Retest", "Report Query", "Print", and "Send to PACS" (for Windows software) operations.
Report Settings:
Click the "Report Settings" button on the "Function Settings" interface to enter the report settings interface. Here, you can input hospital information, report notes, and select the default printer (for Windows software).
In the report settings interface, users can choose whether to display bone physiological age, height prediction, predicted age of osteoporosis, doctor's signature, contact number, and header icon in the report.
Note: The above description is excerpted from the product manual.
Medical Device Name: Wireless BMD
Medical Device Manufacturer: Nanjing Kejin
Medical Device Registration Certificate No.: SuXieZhuan 20222071058
Medical Device Advertisement Approval No.: SuXieGuangShen (Wen) No. 251221-12857
Advice: Please read the product manual carefully or purchase and use under the guidance of medical staff. For contraindications or precautions, see the manual.